By Carissa Wong, PhD Cancer Immunology
The 2oth annual World Cancer Day is on February 4th. Here, Bristol PhD student and describes their research that aims to help the body use its own defence system against cancerous cells.
Someone is diagnosed with cancer every two minutes in the UK. Cancer is the second leading global killer and led to 9.6 million deaths worldwide in 2018. It develops when cells, the building blocks of your body, reproduce too rapidly, often forming a lump of cancer cells called a tumour.
Your body has an in-built defence against tumours called the immune system. The immune system is a group of different cell types that travel throughout your body, on the look-out for abnormal cells such as germs or cancer. Once a threat has been identified, the immune system tries to eliminate it.
So why do tumours grow? A tumour is a jam-packed battleground where the fight between cancer and the immune system determines whether a tumour shrinks or expands. In solid tumours, cancer cells can manipulate their surroundings to hide from and hinder the anti-tumour immune response, forcing the immune system to surrender. My research aims to unpick how cancer cells strategise to win; understanding cancer’s tactics will allow us to exploit them.
If a tumour grows, treatments are required. Surgery to remove the tumour is often not enough so radiation (radiotherapy) and toxic chemicals (chemotherapy) are used. Nasty side effects of radiotherapy and chemotherapy involve damage to non-cancerous tissue. Firing radiation towards a tumour will damage healthy tissue along the way, while chemotherapy targets all rapidly replicating cells regardless of whether they are cancerous, leading to side effects such as hair loss.
But what if there was a more specific way of targeting cancer that could provide long-lasting protection from the disease and reduce the reliance on traditional therapeutics? Immunotherapy could be the answer. This involves helping the immune system to recognise and destroy cancer. Immune cells can target cancer cells with high accuracy and have a remarkable ability to ‘remember’ what cancer looks like.
| Bristol student develops app for cancer community after battling the illness
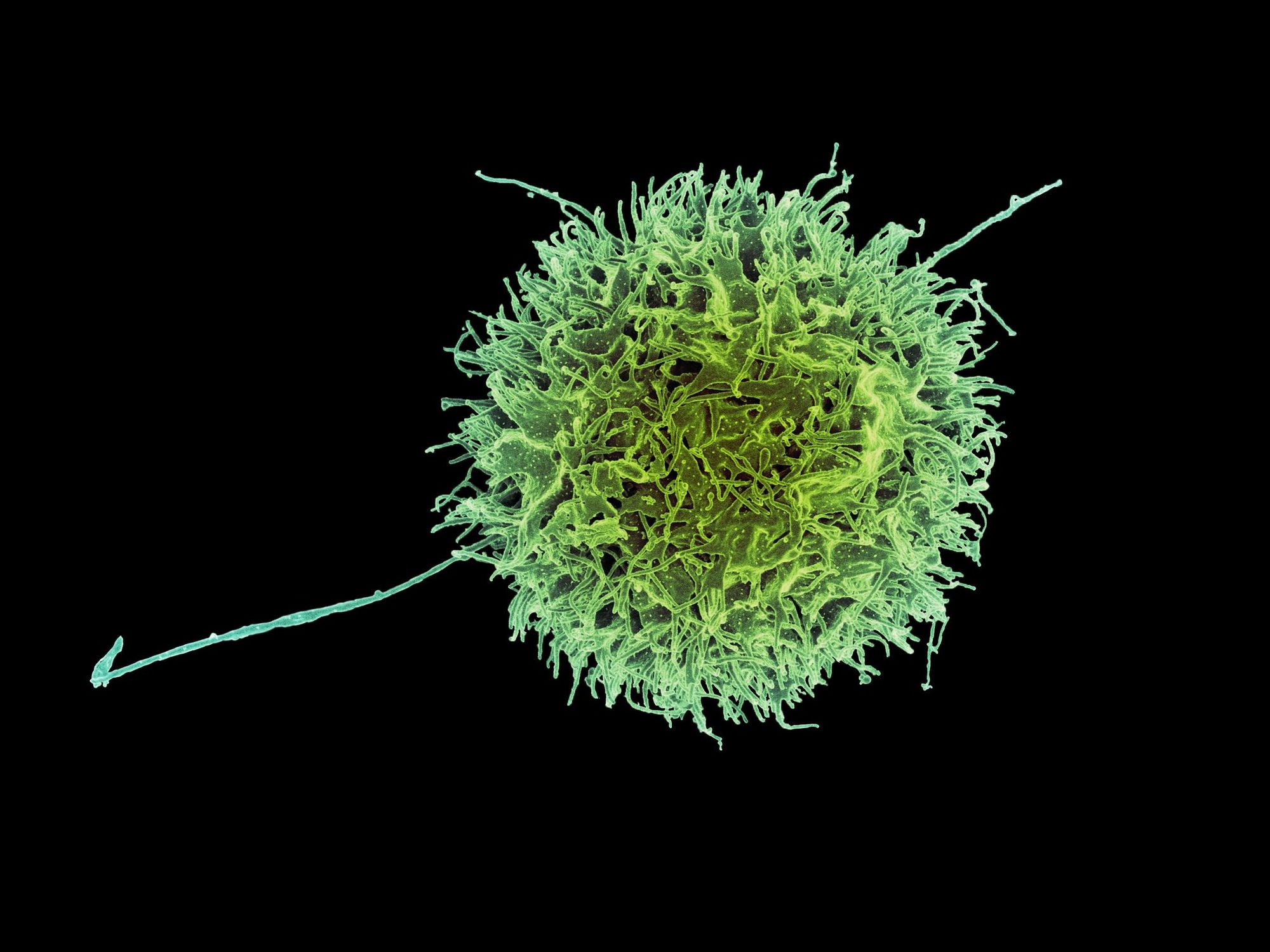
The key players of the immune system are T-cells which are adept at detecting cancerous traits when normal cells turn bad. The three main types of T-cells are the helper, regulatory and killer T-cells. Killer T-cells can recognise and destroy cancer cells. Helper T-cells assist their killer counterparts to successfully do their job. If killer T-cells kill too well, it can result in unintended damage of healthy tissue; regulatory T-cells moderate the actions of killer T-cells to prevent this.
Research in our labs has found that a T-cell surface molecule seems to increase the number of regulatory T-cells and decrease the number of killer T-cells in mouse tumours. Importantly, my colleague has found that the molecule can act to increase tumour growth. This begs the question: does it support tumour growth by changing the way T-cells invade tumours, or reducing the killing ability of the killer T-cells?
Mouse models are an invaluable tool to develop cancer immunotherapies because mice have a very similar immune system to us. At the same time, scientists are constantly trying to minimise the number of animals required to further our understanding of disease.
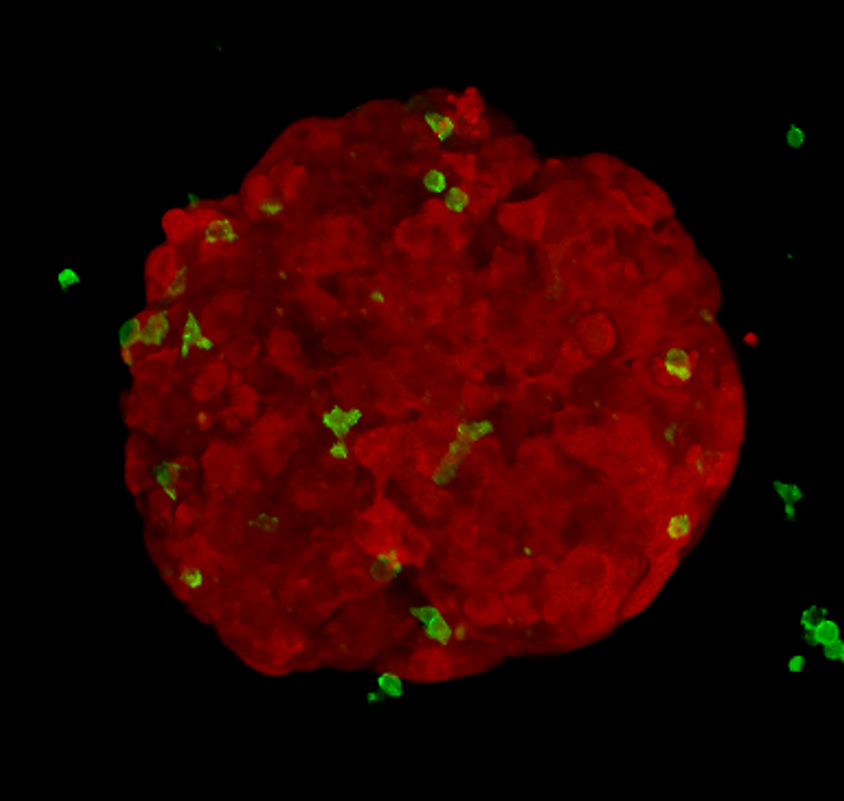
Alongside animal models, researchers grow cancer cells in plastic flasks and plates. My research project involves growing cancer cells into compact spheres made of hundreds of cells, called tumour spheroids. I add killer T-cells to these spheroids and image what happens. This method is cheap, quick and reduces animal usage.
I can label different cell types with different colours; using a microscope, I image red spheroids (which look like tiny raspberries), green killer T-cells and blue dead cancer cells over time. In order to gain meaningful information, I’ve developed an automated method to extract measurements from the pictures I acquire. This allows me to objectively answer key questions: how do tumour-derived molecules affect the number of killer T-cells entering tumour spheroids? How do they affect how deeply T-cells penetrate? Do they change how well the T-cells kill?
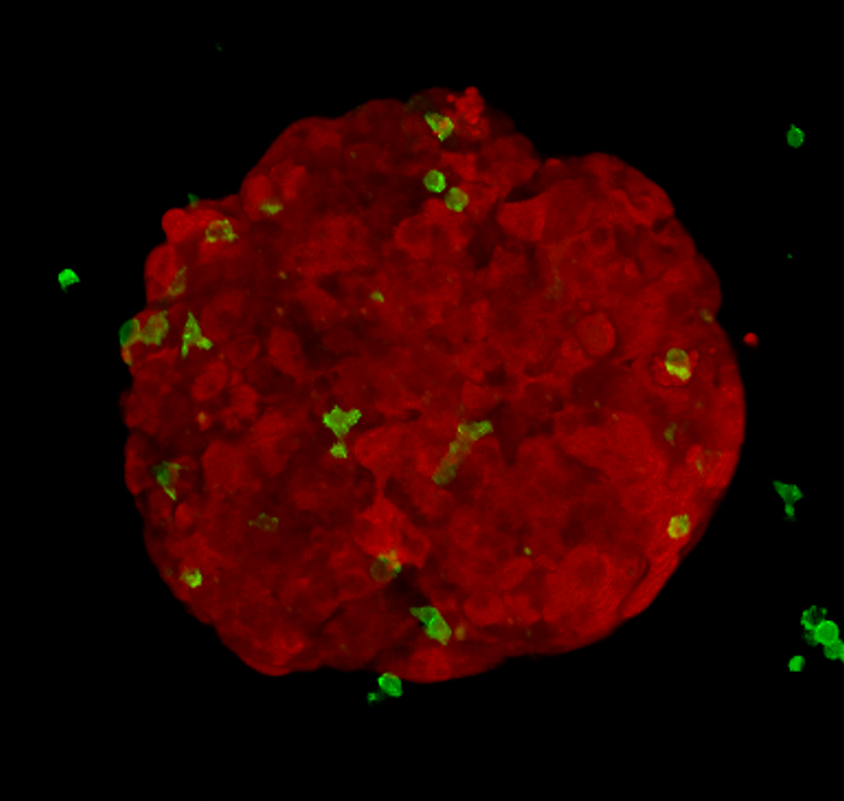
If I find that a molecule, similar to the one previously discussed, changes the way killer T-cells interact with tumour spheroids, I’ll work to decode the messages it is sending to the killer T-cells and investigate whether this tumour-talk can be altered.
| Lessons on tissue repair from fruit flies
For some, immunotherapies have been a life-saving option when traditional treatments have failed. Unfortunately, the current range of immunotherapies is limited and only helps a small proportion of patients. These immunotherapies are also often associated with side effects that can be worse than those of chemotherapy or radiotherapy.
We need more immunotherapeutic options, and we also need to improve them by deepening our understanding of how they work. Our lab is working on this problem by aiming to pinpoint new ways to boost the cancer-fighting immune-weapons within us, with the long-term goal of making new and improved immunotherapies available. Beat cancer? Yes we can.
Featured image: Epigram / Carissa Wong
Want to write about your research? Get in touch!