By Sophie Holman-Jones, Third Year, Biomedical Sciences
Researchers at the University of Bristol have successfully manipulated cell-like micro-bots in the form of synthetic protocells to generate nitric oxide (NO) gas in blood vessels of rabbits. Developing micro-bots capable of integration within human cells could give rise to many possibilities with clinical benefits in the near future.
When biomedical engineering was in its infancy, the possibility of the use of artificial models (bots) to regulate disease management would have seemed impossible. This month, thanks to a team from the University of Bristol, we took a step closer to making this a reality. The ground-breaking research was headed by Professor Stephen Mann and Dr Mei Li, from the Department of Chemistry, in collaboration with colleagues from Hunan University and Central South University in China.
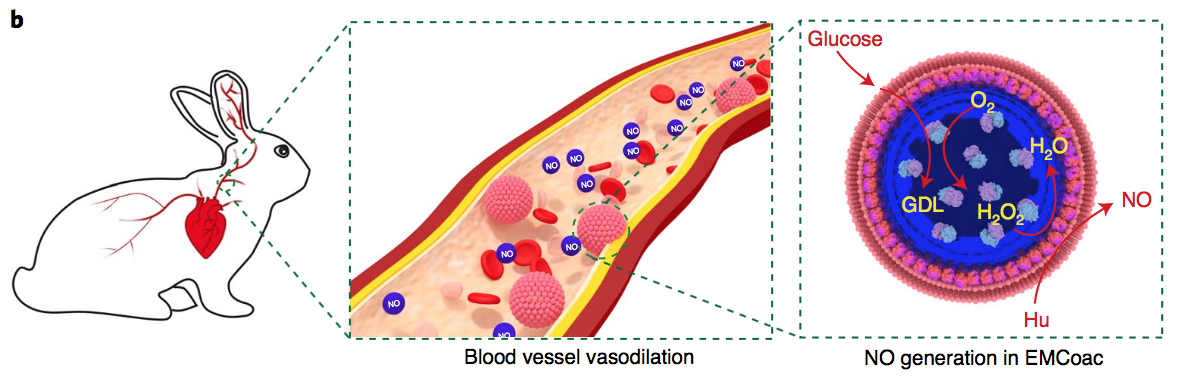
The team innovatively managed to develop bots (which they refer to as ‘protocells’) by deriving fragments of red-blood cells from sheep blood and manipulating them to assemble themselves around tiny droplets of an enzyme known as glucose oxidase. After assembly, the protocells were injected into rabbits, which is where a series of chemical reactions was observed.
In the presence of glucose, which occurs naturally in the blood, the enzyme glucose oxidase produces the chemical compound hydrogen peroxide. Red blood cells also contain a protein called haemoglobin, which mainly functions to transport oxygen around the body. In this case, haemoglobin recruits the hydrogen peroxide in order to aid the break-down of hydroxyurea (an oral drug commonly prescribed for certain cancers) into NO. The production of NO via this series of reactions is extremely rapid and was shown to increase twenty-one-fold in ten minutes.
The production of NO increased twenty-one-fold in ten minutes
NO is naturally produced in the body in order to relax smooth muscle and thus stimulate blood vessel dilation. The profound effect of protocell injection on NO production in sheep caused a 1.2-fold increase in blood vessel diameter compared to an unrelaxed vessel.
Mann’s team also noted a significant reduction in heart rate and blood pressure. The effect of blood vessel size can be thought of similarly to drinking through a straw; the smaller the diameter of the straw, the harder you need to suck to drink through it. Likewise, the wider the vessel diameter, the less force needs to be generated by the heart to pump the blood around the body.
Cardiovascular disease is the single biggest killer globally and costs the NHS £7 billion each year.
High blood pressure is associated with a plethora of health complications, including heart attacks, strokes and heart failure. According to the World Health Organisation cardiovascular disease is the single biggest killer globally and costs the NHS £7 billion each year. The reduction in blood pressure seen by Mann’s team in rabbits means that a similar effect could potentially be replicable in humans. This method could have real therapeutic potential, in terms of preventing health problems associated with high blood pressure and relieving some of the financial burden on our health systems.
Even more exciting are the many other potential applications this pioneering work could have in the wider clinical field, which have not yet been elucidated. For example, it could allow for drugs to circulate in the body for extended periods of time or be administered to specific therapeutic targets that are otherwise difficult to reach via conventional methods. Medical drugs could also be designed to be more compatible with the patient to ensure they are more tolerable by the body.
Genetically modified viruses could be the key to treating chronic kidney diseases
Bristol research reveals how cancer puts brakes on the immune system
In short, micro-bots could be an invaluable tool to manage cardiovascular disease and many other related illnesses. Using bots in this way is still in its early stages and should be corroborated by other studies before extensive clinical use. Nevertheless, this is still an exciting prospect and certainly one to watch for in the field of biologics.
Featured Image: Flickr / Mike Goad
Which new promising clinical therapies have you heard about? Can protocells be an answer to many critical illnesses?