By Carissa Wong, 3rd Year PhD, Cancer Immunology
A study led by the University of Bristol has revealed a new way of utilizing the drug Pembrolizumab to help in the fight against cancer. The research, published in Science Signaling, outlines how the antibody can aid the cancer-killing soldiers of the immune system, T-cells.
Pembrolizumab is a form of immunotherapy, a treatment which boosts cancer-killing immune responses in the body. Although the drug has been approved to treat severe skin cancer since 2015, past clinical trials showed that the drug often cures fewer than 50 per cent of patients.
A limitation to the effectiveness of the drug is that, although scientists know it strengthens T-cell response mechanisms once inside the tumour, they don’t know how exactly it interacts with T-cells to achieve this.
Part of the problem is that ‘we don’t have a very good idea of how the behaviour of cancer-killing T-cells is changed inside a tumour,’ explains lead researcher Professor Christoph Wülfing. ‘If we don't know in detail how tumours inhibit T-cells to start with, it’s hard to imagine how the drug reverses this inhibition.’ Building a better picture of how the drug alters T-cell behaviour inside tumours makes it easier for scientists to figure out how to amplify useful effects.
The researchers found that T-cells taken out of mice with cancerous tumours were weaker cancer-killers than the T-cells extracted from healthy mice. In the same way that a heavy night of drinking leaves you with a hangover, T-cells extracted from tumours remained intoxicated by the tumours’ chemicals for some time after they had stopped consuming them. What they found next was surprising.
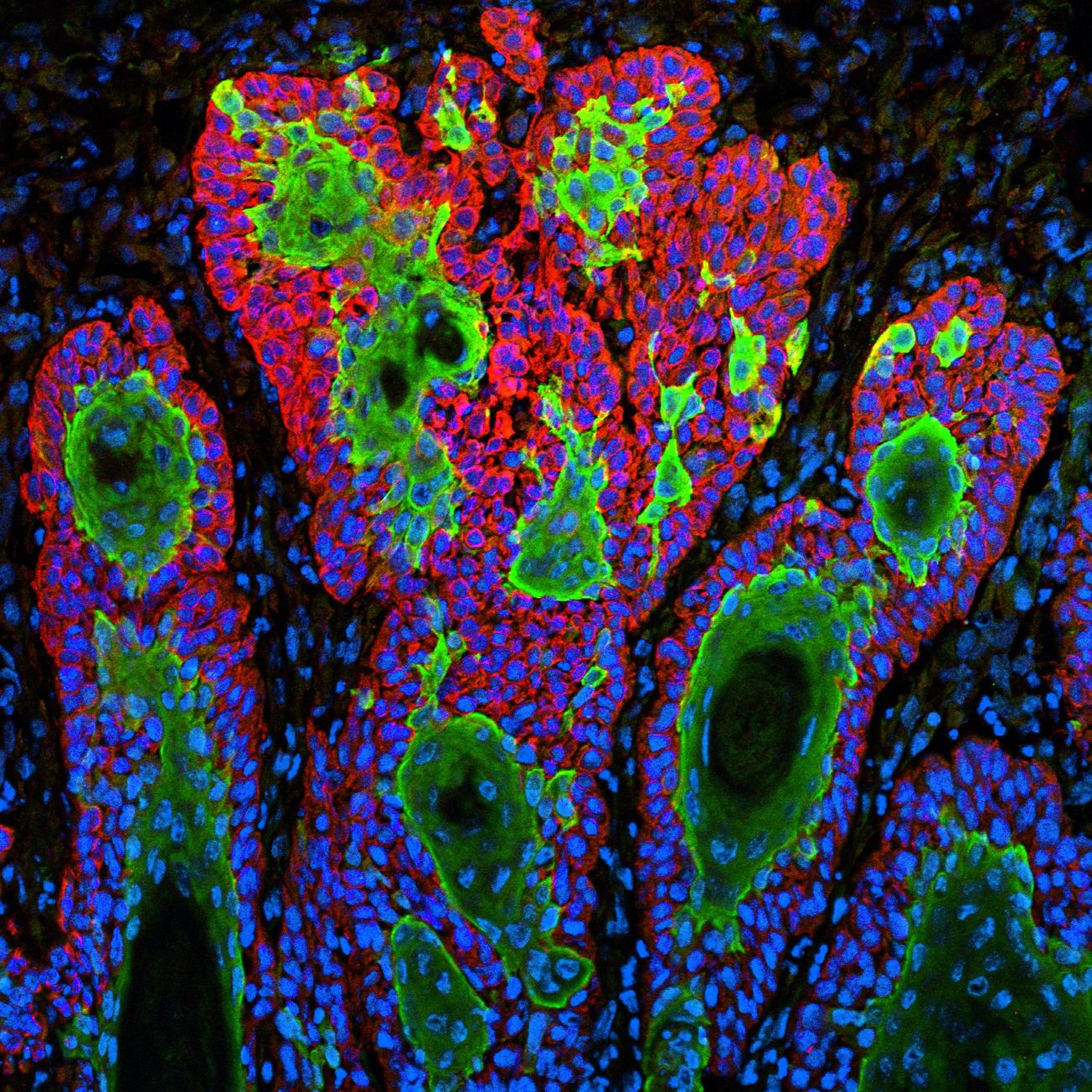
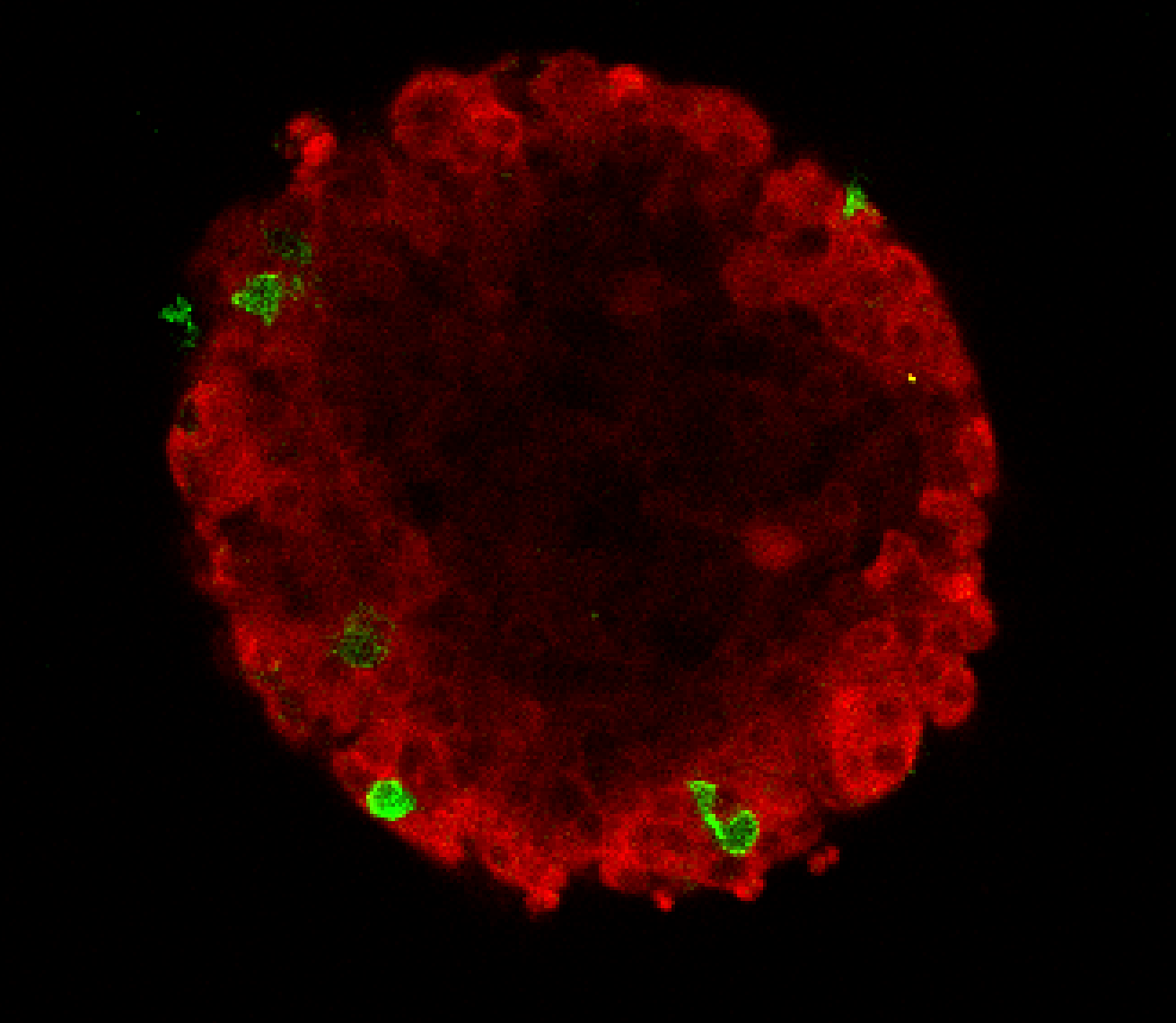
Imagine two bubbles floating and then fusing together on one side. This is what it looks like when a cancer-hunting T-cell latches onto its target. The T-cell and the tumour form an area of fusion between the two, called the synapse.
‘If we don't know in detail how tumours inhibit T-cells to start with, it’s hard to imagine how the drug reverses this inhibition.’
To kill the cancerous tumour cell, the T-cell fires toxic chemicals through the synapse into it. The researchers noticed that T-cells from healthy mice fused tightly to one spot of a cancer cell, while T-cells from the tumours of the cancerous mice struggled to bind and stay in one place. This is when the Bristol researchers had an interesting idea.
To test whether the drug restores the ability of T-cells to hold on to cancer cells, thereby making them better cancer-killers, tumours were grown in mice and some mice were given a medicine, which was the mouse-equivalent to Pembrolizumab. Although the drug did not completely reverse the defect, it substantially improved the ability of T-cells to hold on to and kill cancer cells.
To work out why exposure to a tumour made a T-cell lose its grip, the scientists compared the skeletons of T-cells obtained from mouse tumours and healthy mice. The skeleton of a T-cell controls its movement in the same way our skeleton enables us to walk and stretch. However, unlike ours, the T-cell skeleton has the ability to collapse and re-build in different parts of the cell.
Rather beautifully, when a T-cell from a healthy mouse fused to a cancer cell, its skeleton rearranged to form a ring, which outlined the edges of the junction between the two bubble-like cells. Crucially, a skeleton-free zone at the centre of the fused area enabled the rapid transfer of cancer-killing chemicals from the T-cell to the cancer cell.
A T-cell taken from a tumour of a cancerous mouse failed to maintain a skeletal ring around the fused area. Instead, its skeleton formed a messy mesh across the entire joined region. There was no skeleton-free zone in the middle.
This could explain why these T-cells struggled to hold on to their target; it would be like trying to grip a football with only a couple of fingers. It also explained why these T-cells did not kill properly. They simply had no clear window through which to freely fire ammo at the cancer target.
Yes we can-cer: shrinking tumours by boosting the immune system
University of Bristol spin-out wins £1.1 million to develop new cell therapies for solid cancers
Does the drug give T-cells a firmer hold on cancer cells by correcting their skeletons? When they looked at the skeletons of T-cells from tumours which had been exposed to the drug, it was a breakthrough moment. T-cells from tumours that received the drug regained the stable skeletal ring at the synapse when they bound to cancer cells. They also cleared their skeleton from the synapse centre which helped them to kill better.
‘This study shows how a series of steps is required to enable a T-cell to kill a cancer cell and if these steps become less efficient, they add up to a substantial overall defect in the killing power of T-cells,’ says Wülfing.
Looking forward, scientists continue to search for new strategies to improve the therapeutic potential of the drug. Immunotherapies can be lifelines, but they don’t work for everyone. If we want immunotherapies to succeed for more patients, knowledge is power: to shrink lumps and save lives.
Featured Image: Unsplash / ZEISS Microscopy
Are you optimistic the research by Bristol scientists will yield a breakthrough in cancer treatment?