By Lucy Mahony, Fourth Year, Bioinformatics
Researchers from the University of Bristol have built microbial ‘micro-reactors’ capable of producing carbon-neutral hydrogen. This exciting discovery takes us one step closer to sustainable fuel production.
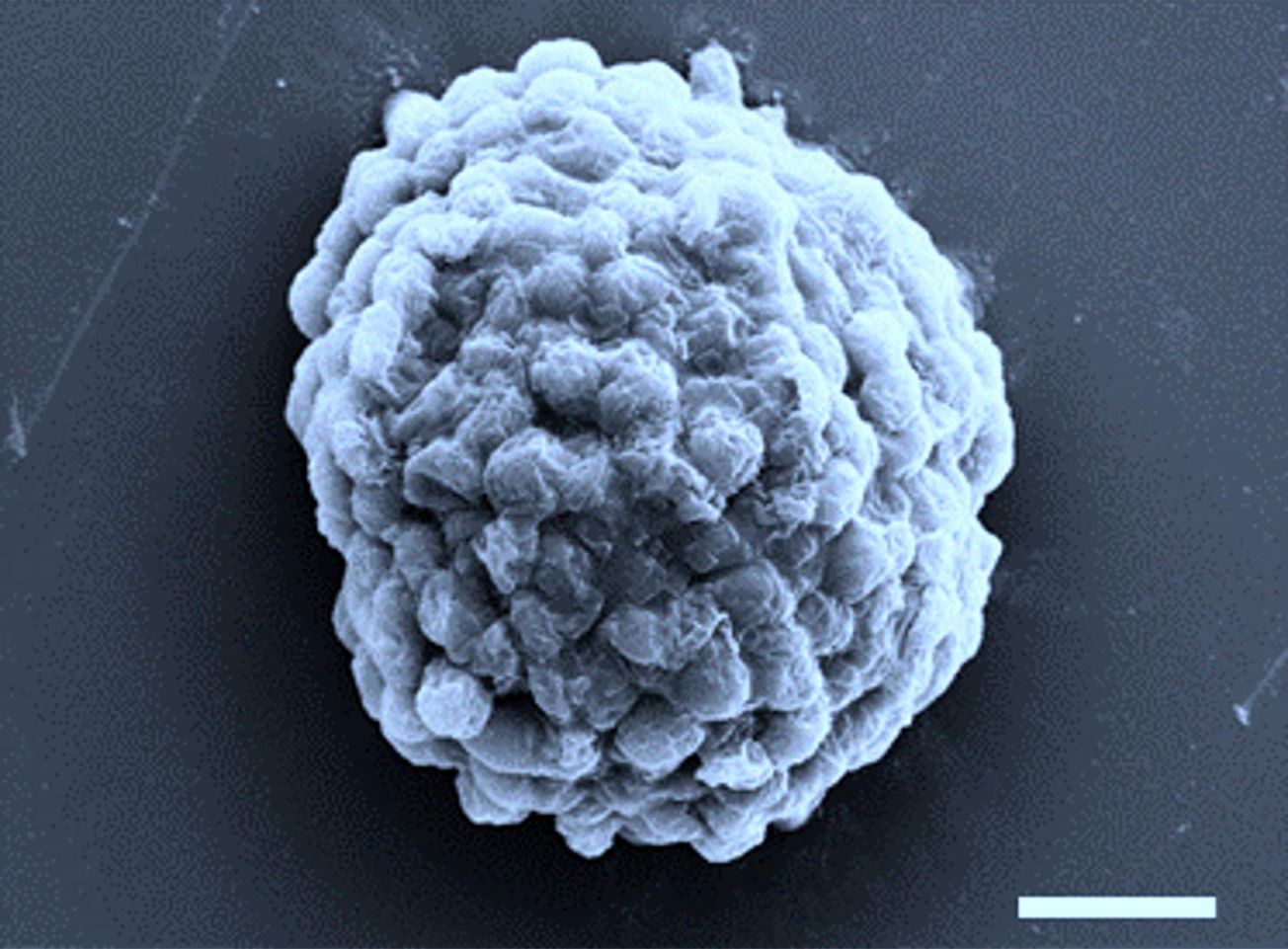
The study published last month in Nature Communications in collaboration with the Harbin Institute of Technology, China, describes how micro-droplets filled with algae and coated with bacteria were created as miniature hydrogen generators.
These microscopic living factories – around ten times the size of a red blood cell - produce hydrogen in the presence of light, rather than oxygen as algae usually do, using the energy in the light as opposed to the vast amounts of electricity used in current hydrogen production methods. This hydrogen, which is renewable and produces no greenhouse gasses, can then be piped off and used in hydrogen fuel cells, possibly one day to fuel our cars.
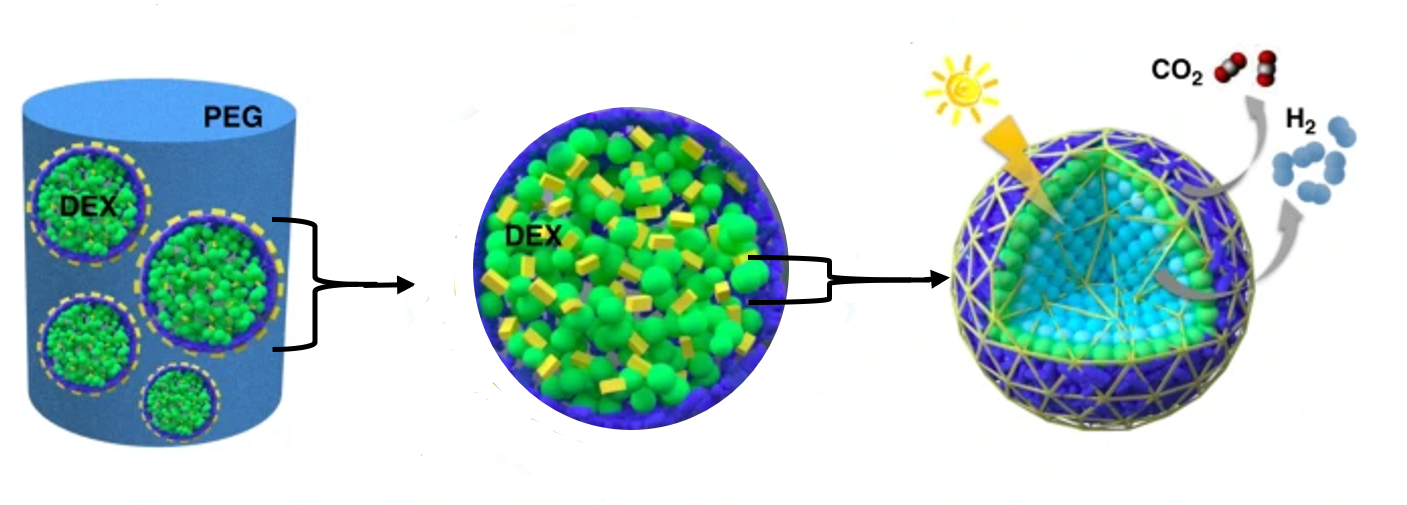
To build these micro-reactors, the algal species Chlorella pyrenoidosa and the bacterial species Escherichia coli were cultured in the lab. They were then mixed with a solution of two chemicals, dextran and PEG, to form droplets which trap the algae and bacteria inside them spontaneously.
These droplets can then be transferred into a more concentrated PEG solution which draws the water out of the droplets. As a result, the droplets shrink and become tightly packed making the centre of the sphere becomes a ‘micro-niche’ where different chemical and biological conditions can exist compared to the outside solution.
The algae and bacteria survive this process, and the outer bacteria use up the available oxygen in respiration. This starves the centre of the sphere of oxygen. The oxygen-deficient environment results in the centre algae producing hydrogen as a product of photosynthesis, instead of oxygen.
This proof-of-concept research lays the foundations for future research by providing a relatively easy and highly modifiable method. Combining this method with other bioengineering approaches, such as the use of genetically or chemically modified algae, has the potential to improve the yield of hydrogen produced.
The structure of moth wings reveals the secret to how they outsmart bats
Pterosaurs: how did these giant lizards get better at flying?
Indeed, one of the authors – Co-Director of the Max Planck Bristol Centre for Minimal Biology at Bristol – Professor Stephen Mann, has already stated they ‘hope to develop future work.’ Professor Xin Huang at Harbin Institute of Technology added that the method is ‘facile and should be capable of scale-up’ and is also flexible for making other chemicals like ethanol.
This research is significant for a second reason. Creating groups of cells that automatically assemble themselves into a specific structure has always been a big challenge in synthetic biology. The researchers have overcome this issue by creating micro-droplets to selectively hold and organize different cell types in miniscule ‘packages’. This technique could have significant biomedical applications in cell therapy and tissue engineering.
Featured Image: Harbin Institute of Technology / Prof Xin Huang