By Genevieve Panton, Third year, Biochemistry
CRISPR-Cas9, a groundbreaking gene-editing technology discovered in 2012 and awarded the Nobel Prize in 2020, has recently achieved a significant milestone with the approval of the first CRISPR-Cas9 drug, Casgevy.
This landmark approval, initially granted in the UK for the treatment of sickle-cell disease and β-thalassaemia in November 2023, was followed by the US with Food and Drug Administration (FDA) approval for sickle-cell disease treatment in December 2023. The European Medicines Agency (EMA) has recommended Casgevy for approval and will finalise its decision next year.
Sickle-cell disease (SCD) and β-thalassaemia, both caused by genetic mutations affecting oxygen delivery to tissues, have long presented challenges in treatment. A bone marrow transplant has previously been the only cure for SCD, and β-thalassaemia often requires monthly blood transfusions. Casgevy, made by Vertex Pharmaceuticals and Crispr Therapeutics, targets the genetic mutations responsible for these conditions.
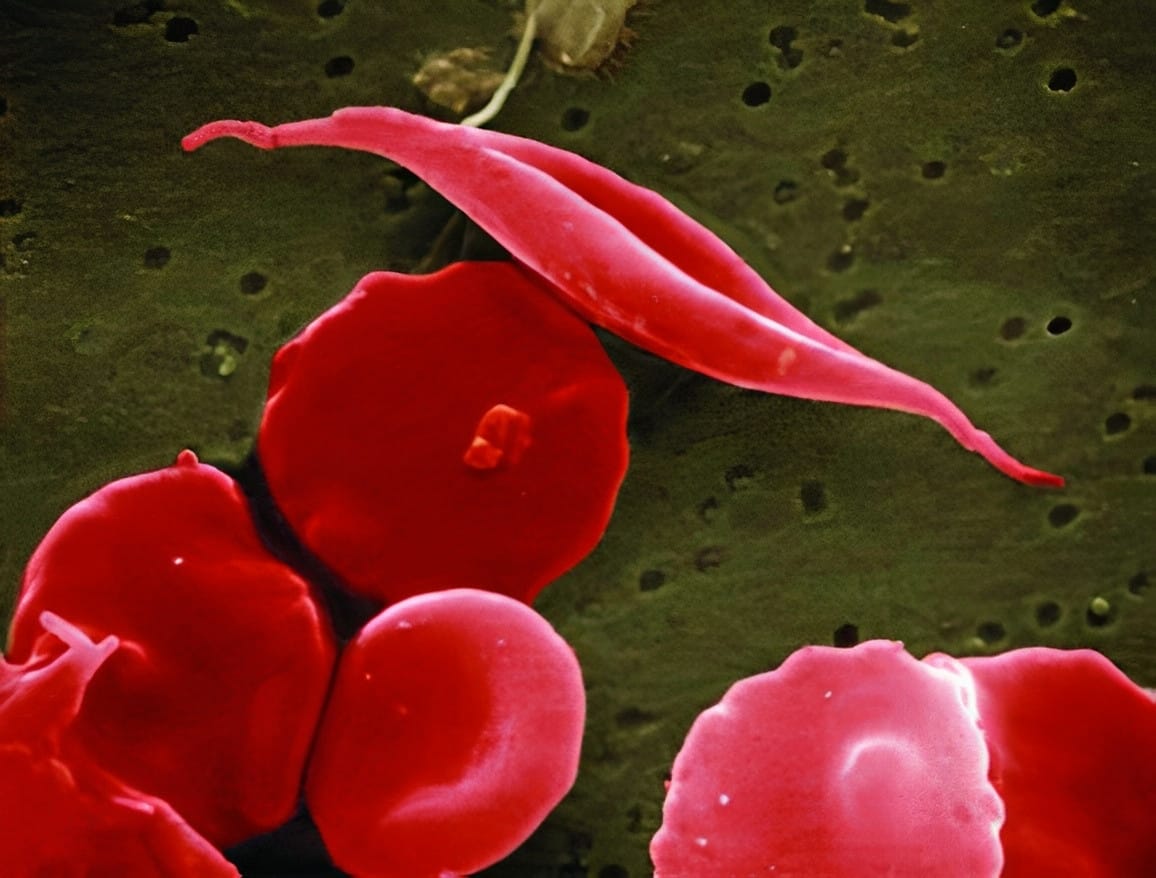
Genetic mutations can lead to diseases, because genes contain the instructions necessary for building proteins. When these instructions are faulty, the resulting proteins may not function as intended. In the case of SCD, there's a mutation in the gene responsible for producing haemoglobin. Haemoglobin plays a crucial role in carrying oxygen within red blood cells. However, when haemoglobin is synthesised incorrectly, red blood cells adopt a distinctive crescent shape. As these abnormal cells move through blood vessels, they tend to clump together, slowing down blood flow and reducing the supply of oxygen to tissues. This process triggers episodes of severe pain referred to as 'pain crises,' significantly impacting a patient's quality of life and potentially leading to other health complications, some of which can be fatal. Similarly, β-thalassemia results from a mutation that reduces the production of haemoglobin, leading to anaemia, osteoporosis and can cause heart and liver problems.
CRISPR-Cas9 is like a pair of scissors. Patient’s stem cells are taken out of their bone marrow and edited in a lab using Casgevy, which works by cutting and disabling the BCL11A gene. This gene normally prevents the production of a type of haemoglobin found only in fetuses. By turning off this gene, the body can generate fetal haemoglobin. Unlike the adult version affected by SCD genetic mutations, fetal haemoglobin is not impacted. The edited cells are then put back into the patient’s body, resulting in the production of a functional form of haemoglobin, leading to better oxygen flow. Extending successfully to β-thalassaemia patients as well, this therapy removes the need for frequent blood transfusions or life-threatening transplant procedures, massively improving quality of life.
While the approval of Casgevy is cause to be optimistic, the widespread use of the drug is still in its infancy. Like most gene editing treatments, it comes with a hefty price tag, approximately $2m per patient in the US. The UK has yet to determine the drug's cost. For now, the global reach of Casgevy remains limited to high-income countries until it becomes more affordable and accessible.
Despite Casgevy’s proven safety thus far, CRISPR technology remains controversial due to its potential to edit genomes in an unknown way, known as 'off-target' effects. Like all new drugs, patients treated will be monitored closely in the coming months, but crucially, Vertex Pharmaceuticals will follow the progress of patients for the next 15 years to determine the long-term safety of the gene-editing drug. These concerns have been around since CRISPR-Cas9’s discovery. Notably, ethical considerations have arisen regarding the potential application of CRISPR-Cas9 in editing human embryos. The elusive nature of the long-term consequences of genetic editing, extended to the offspring of these subjected individuals, raises ethical questions around the issue of informed consent.
Casgevy's approval will undoubtedly fuel the ongoing debate surrounding the present and future of gene editing. Despite accessibility and safety concerns, this approval signifies a remarkable achievement in biotechnology with the potential to improve the lives of many people and their families.
Featured image: Flickr / NIH Image Gallery
Do you think gene-editing drugs are a step too far?