By Helen Barber, Second Year, Biochemistry
The exact time for which complex life emerged has long eluded researchers in their quest to track the evolutionary timeline of life on earth. Previous studies suggest that complex life began anywhere between 800 million and 1,800 million years – a very broad range of time. Now, ground-breaking research conducted by the University of Bristol has discovered a method to make this estimated time range more accurate.
The University of Bristol’s School of Earth Sciences has discovered that eukaryotic organelles decay at slow enough rates to undergo fossilization, meaning they may be able to help pinpoint the beginnings of complex life. Eukaryotes are one of the three major domains of life; along with Archaea and Bacteria, they consist a major and distinct branch of the ‘tree of life’.
Unlike Archaea and Bacteria, termed as ‘prokaryotic’ forms of life, eukaryotes are distinctive due to the more complex nature of their cells. Animals, plants and fungi are all examples of eukaryotic life.
What makes eukaryotic cells distinctive is that they contain membrane-bound organelles not found in prokaryotes, such as the nucleus, chloroplasts or the well-known ‘powerhouse of the cell’ – the mitochondria. These organelles can be viewed as the ‘organs’ of the cell, each with a specialised role to play for proper cell function. By evolving to have these roles compartmentalised using membranes, rather than be free-floating like in the prokaryotic cells of the Archaea and Bacteria, eukaryotes can achieve more complex lifeforms.
Previously, it was believed that these specific eukaryotic organelles decayed too quickly after a cell’s death to become fossilized. This is important as fossils are the major method used by researchers to reconstruct the evolutionary timeline.
By evolving to have these roles compartmentalised using membranes eukaryotes can achieve more complex lifeforms
‘The evolution of eukaryotes was a hugely important event in the history of life on Earth, but fossils of these cells are difficult to interpret’, explains one of the study’s co-authors, Professor Phil Donoghue, Professor of Palaeobiology. ‘Some of them have structures that could be organelles, but there’s long been this assumption that organelles cannot be preserved because they would decay too quickly.’
Without preserved organelles, it has historically been difficult to distinguish early single-cell eukaryotes from bacterial or archaeal cells. Other commonly used methods, such as looking for highly complex cell walls and larger cell sizes, are unreliable. Often, cell walls may have degraded over time, and in rare instances, bacterial and archaeal cells can grow to the sizes which are associated with eukaryotes. This leaves an area of uncertainty with both methods.
It has historically been difficult to distinguish early single-cell eukaryotes from Bacterial or Archaeal cells
New, more reliable methods are greatly needed in order to solve the controversies surrounding the origin of eukaryotic cellular life. The new study, co-authored by Professor Donoghue and PhD Emily Carlisle, may help open up these much-needed new avenues of identification. Emily Carlisle watched and studied exactly how the eukaryotic organelles changed as they decayed. Emily describes how she ‘spent several weeks photographing algal cells as they decayed, checking the condition of the nuclei, chloroplasts and pyrenoids.’
From these observations Carlisle was able to conclude that the ‘organelles don’t decay immediately after cell death’ – as previously thought – but instead can ‘take many weeks to dissolve’. Though a few weeks may sound short when compared to the millions of years existing between us and these pre-historic eukaryotic cells, this is long enough for the organelles to be preserved and even seen within fossils.
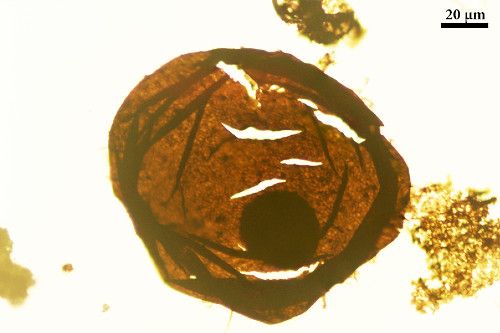
Researchers at the University’s School of Earth Sciences have already contributed new insights using this information. Shuiyousphaeridium (seen in the image below), is a fossil dated back to 1,700 million years ago. It has long been controversial in the research community due to its ‘nuclei-like structures’.
Some believe that these structures only look like nuclei, meaning in theory they still classify the Shuiyousphaeridium as a prokaryote. Others, however, disagree, believing these structures are the fossilized remainders of nuclei, classifying the organism as an early example of a unicellular eukaryote.
The Shuiyousphaeridium fossil is an early example of eukaryotic life
However, the latter interpretation that Shuiyousphaeridium is a eukaryote has been largely rejected. Dr John Cunningham, a lecturer at the School of Earth Science, explains: ‘This interpretation has previously been dismissed because of the assumed rapid decay of nuclei’.
This new Bristol study sheds new light on this interpretation, leading Dr Cunningham to believe that ‘the structures in Shuiyousphaeridium are likely to be nuclei’. This makes the Shuiyousphaeridium fossil an early example of eukaryotic life, providing much needed new insight into the origin of complex life.
Pterosaurs: how did these giant lizards get better at flying?
University of Bristol study explores the truth behind zebra stripes
The new classification of Shuiyousphaeridium places the origin of complex eukaryotic life to at least 1,700 million years, which is on the older end of the previously offered broad time scale (800 to 1,800 million years). Complex life may have begun much earlier than previously believed, using this time to evolve into the millions of animals, plants and fungus we see today.
The team at the University of Bristol hope that using this new insight on organelle decay, more fossils may be correctly identified and placed within their correct domain. This will allow for a more accurate construction of the tree of life and help to uncover the evolutionary timeline of eukaryotic cells – a timeline that has led, finally, to us.
Featured Image: Unsplash / Gabi Scott
Can you believe that our ancestors used to be tiny, single cells?









