By Alex Lawrence, MSc Palaeobiology
New research has found that increasing the complexity of cellular switches can greatly increase the precision with which a cell’s biochemical processes can be controlled. This could lead to higher yields in the industrial manufacture of drugs, medicine and food.
The study, which was published in Science Communications, was led by University of Bristol PhD student Veronica Greco, who was joined by colleagues from the School of Biological Sciences and the Max Planck Institute for Terrestrial Microbiology. Their findings have potential implications for the field of synthetic biology and how cells are designed for use in biomanufacturing operations.
Bioengineering is becoming increasingly important in the production of food, drugs and other chemical products. The industry uses genetically engineered single-celled microorganisms to produce vast quantities of useful products, such as insulin, antibiotics and preservatives like citric acid. However, to do so efficiently, these organisms must be easy to control.
This is particularly important in managing the timing of distinct phases throughout the production process. The first phase is growth, then, when the number of cells is high enough, the process moves onto the ‘production phase’. Bioengineers must design cells that can easily be switched from one phase to the next, and also be able to precisely control how much of the antibiotic, vitamin, or other chemical they produce.
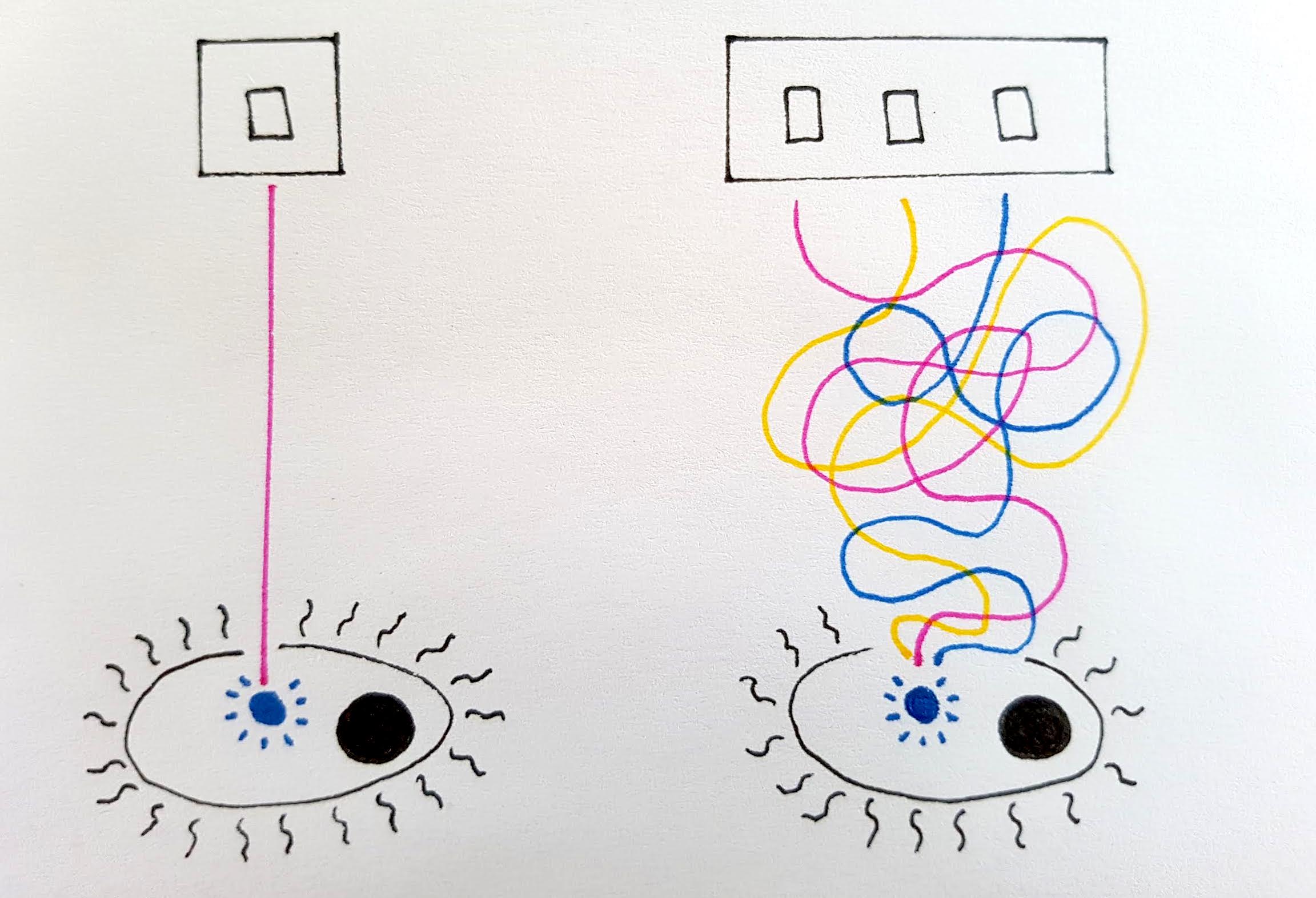
This is generally done by creating cells which respond to a stimulus, such as light levels or a particular molecule, which acts as a switch to turn the cell ‘on’ or ‘off’. With this in mind, cell control is designed to be as simple as possible.
Bioengineers only turn ‘on’ the genes within their designed cell which are desired for their product
To turn a cell ‘on’ or ‘off’ as a biomanufacturing unit requires individual genes or groups of genes in that cell to also be turned ‘on’ or ‘off’. Although all cells in the body contain the same genes, not all of them are active in each cell, or even at the same time.
For example, skin cells will not be ‘expressing’ all the exact same genes as your muscle cells. It is these differences which make cells come in all shapes and sizes, each specialized to their function. Bioengineers thus only turn ‘on’ the genes within their designed cell which are desired for their product.
A switch for a bioengineered cell would be able to turn a gene or multiple genes on or off, triggering a change between the growth and the production phase or vice versa. The extent of the stimulus can also control the cell’s level of productivity.
Although in theory this concept would be ideal for giving bioengineers control of cell behaviour, in nature cells are rarely so neat. It is difficult to create a cell where genes are completely turned on or off, and so often enough an unwanted chemical may be produced, which can hinder the production process targeted by the bioengineers and thus reduce efficiency. This phenomenon, known as ‘leaky production’, may be useful in nature but is undesirable in large-scale biomanufacturing operations.
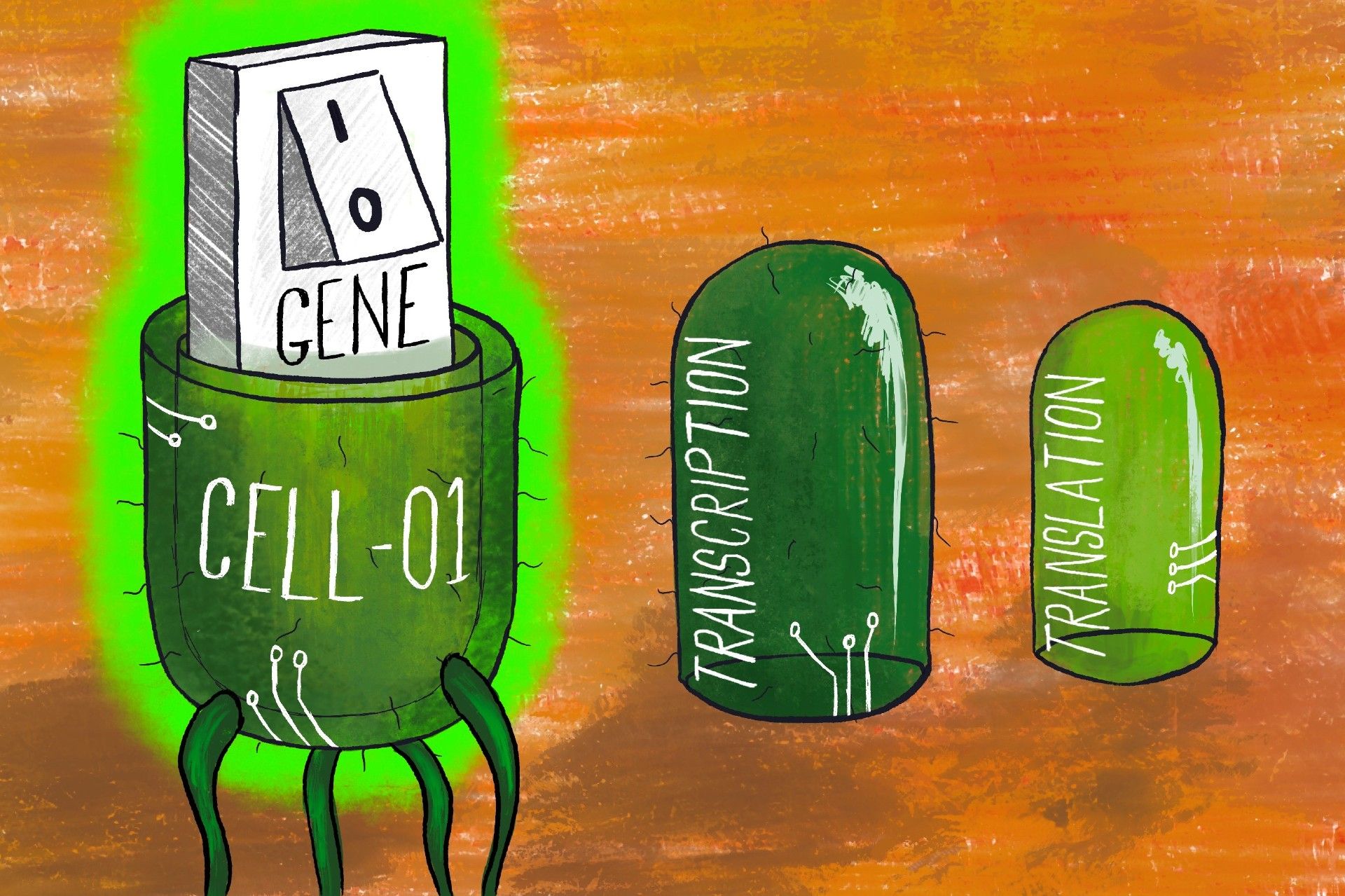
The researchers found that adding switches to the transcription and translation stages of protein production could increase the precision with which genes are turned on and off. Transcription is when the section of DNA that represents a gene is copied into a messenger form called mRNA, which can then be transported around the cell.
This is followed by translation, where the instructions encoded in this mRNA strand are ‘read’, in order to create its corresponding protein. Together, these two processes allow genetic information to be transformed into proteins, which are one of the primary physical components of organic life.
Systems using multi-level switches were more stable and genes could be turned on and off more precisely
Controlling these stages increases control over the cell’s overall biochemical processes, and subsequently the industrial-level fermentation or cell culture necessary for the manufacturing of drugs and medicines. This is a complex, multi-level switch, as opposed to a simple switch which would only target either transcription or translation.
Using mathematical models and computer simulations, the efficiency and levels of control of simple switches to more complex multi-level switches were compared. It was found that systems using multi-level switches were more stable and genes could be turned on and off more precisely.
Veronica Greco explained that the system stability can be compared to that seen at larger biological scales than a single cell: ‘If you look at a Venus flytrap you find that a trap will only close when multiple hairs are triggered together. This helps reduce the chance of a trap closing by accident. We wanted to do something similar when controlling the expression of a gene inside a cell, adding multiple-levels of regulation to ensure it only comes on precisely when we want it to.’
Could a new treatment for diabetic eye disease be on the horizon?
Researchers create carbon-neutral hydrogen from microbial micro-reactors
Application of the study’s findings could allow for more efficient biomanufacturing at an industrial scale, with increased yields of products ranging from penicillin to vitamin C, as well as from plastics to alcohol. Even the production of vaccines, including the COVID-19 vaccines, includes a vital step where bioengineered host cells are grown in a bioreactor.
The number of products we create through biomanufacturing is huge and that number continues to increase as more precise biotechnology, such as this one, advances. The research shows the importance of drawing inspiration from nature and proposes exciting future directions for synthetic biology and bioengineering.
Featured Image: Unsplash / CDC
Sometimes it's good to embrace the complexities of life!