By Miles Gilroy, SciTech Deputy Editor
From the desk of: Mr. Gordon Robson Mackenzie et al. University of Bristol
"My battery is low and it's getting dark." The heart wrenching last words of the Opportunity Mars rover. In the early summer of 2018, a devastating dust storm battered the surface of Mars around where Opportunity was operating. The storm blocked all sunlight from reaching the ground, denying the rover’s solar panels the ability to generate power. Opportunity’s battery slowly drained and the above message was sent back to Earth. After sending over 1000 recovery commands with no success, NASA faced the truth. Opportunity was dead.
It was sad to see Opportunity go, but, fortunately, thanks to a collaboration between scientists at the University of Bristol and the UK Atomic Energy Authority (UKAEA), a breakthrough new form of battery has been successfully created. A ‘diamond battery’ has the ability to provide power for thousands, yes thousands, of years.
💎Scientists and engineers from the UK Atomic Energy Authority (@UKAEAofficial) and the University of Bristol (@BristolUni) have successfully created the world’s first carbon-14 diamond battery.
— UK Atomic Energy Authority (@UKAEAofficial) December 4, 2024
This new type of battery has the potential to power devices for thousands of years,… pic.twitter.com/Kquxpn1PHA
This technology could be put to use in all areas of life and science. As Professor Tom Scott, Professor in Materials at the University of Bristol, said “our micropower technology can support a whole range of important applications, from space technologies and security devices through to medical implants.”
Thanks to the lifetime of these batteries, they will be extremely helpful in the medical sector. Devices such as ocular implants and pacemakers, which require constant power, will not have to be replaced within a human lifetime, reducing strain on medical services and distress in patients.
They could be used to power radio frequency tags, which are used to identify and track devices such as spacecraft or payloads (like Opportunity), reducing the costs of such missions and extending their operational lifespan.

The keen eyed among you may have spotted that Professor Scott used the term ‘micropower’ which makes it sound a bit rubbish. But it’s not. Yes, the term ‘micropower’ means the power generated by a diamond battery is not actually very significant. However, the rate of power output is not what the focus of these batteries is, it’s the lifetime of them. There are countless applications for low power batteries in the modern world, such as the aforementioned medical implementations, but, currently, the batteries used for them don’t last long enough.
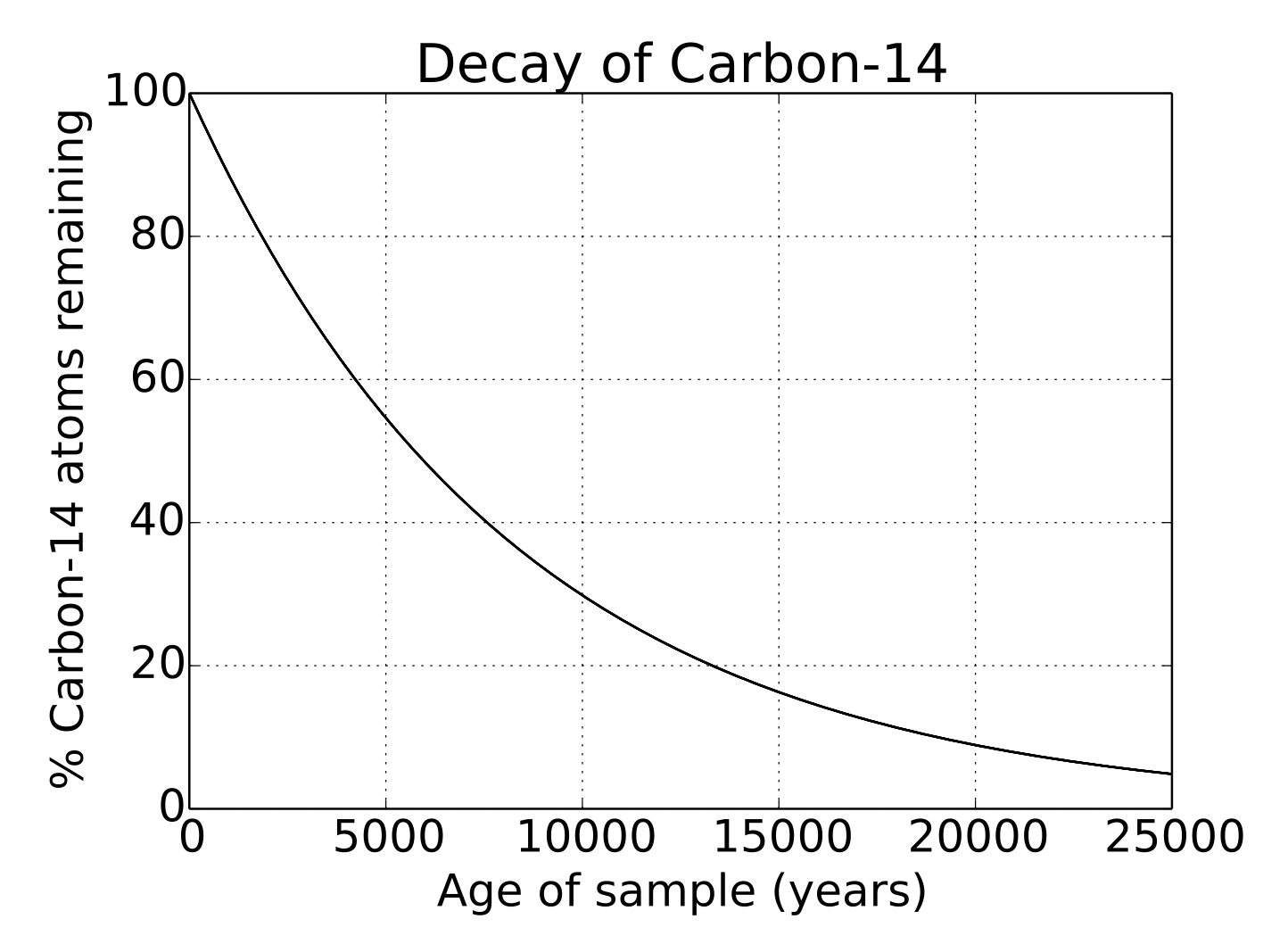
The diamond battery makes use of the carbon-14 (C-14) isotope, the same one used in carbon dating. The half-life of C-14 is roughly 5700 years and it undergoes beta decay, meaning it continuously emits high energy electrons for thousands of years. The battery exploits the energy of these electrons, much like a solar panel exploits the energy of photons, enabling it to produce a low-level, but steady, flow of power for extremely long periods of time.
The benefits of this technology extend beyond its power generation capabilities, as well. The use of C-14 also acts as a safe way of dealing with nuclear waste! C-14 is generated in radioactive graphite blocks in some nuclear fission power plants. By extracting the C-14 from these blocks, they become less radioactive and, therefore, safer and easier to store, reducing the costs of doing so. The UK currently holds nearly 95,000 tonnes of these blocks so we are more than ready to start fuelling these batteries.
Despite this, the diamond batteries are “safe [and] sustainable” according to Sarah Clark, Director of Tritium Fuel Cycle at UKAEA. This is because the C-14 is encased within a diamond (hence the name), meaning no short-range radiation can escape.
While diamond batteries currently only have applications in low power situations, they offer an excellent starting point for the future growth of long lasting batteries.
Featured image: University of Bristol