By Edward Deacon, PhD, Physics
On the 6 October, Professor Dame Sarah Gilbert, the lead scientist in the development of the Oxford Astra Zeneca COVID-19 vaccine, gave a lecture exploring how the vaccine was created. Ed Deacon went to learn more
In the half hour talk, Prof Dame Gilbert discussed the vaccine from its initial inception to its current state and use today.
Using a novel technology platform for the vaccine creation, the University of Oxford scientists were able to create new versions of a vaccine with a modified spike protein easily. The techniques used also enabled some predictive capability in knowing what the side effects of the vaccine would be.
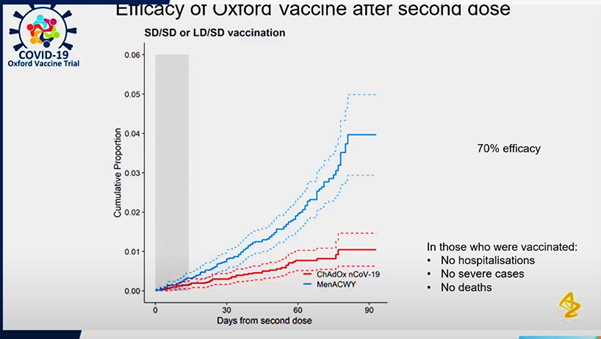
A turning point in its development was the partnership between the University of Oxford and Astra Zeneca.
The ‘most challenging aspect’ in the creation of the vaccine was obtaining and sustaining funding for the work being done by the team at the Nuffield Department of Medicine at the University of Oxford, so the partnership with AstraZeneca - a pharmaceutical giant - was a crucial one.
In addition, the unprecedented circumstances in which the vaccinology department found themselves in meant they needed to work and scale up vaccine roll-out faster than they had ever done before. The manufacturing demands were too great for the University to handle which is why in January 2020, Oxford partnered with AstraZeneca.
The partnership with AstraZeneca - a pharmaceutical giant - was a crucial one
Developers of the vaccine had to work ‘at risk’ meaning processes and trials in the development of the vaccine had to run in parallel, rather than be done in sequence as would typically have been the case. The logistical challenge of planning the Covid vaccine’s creation was one that was highlighted.
As Prof Dame Gilbert described, vaccine developers worked ‘in a different way in order to move on as quickly as possible.’ Nevertheless, the safety of vaccine recipients was kept a priority even with financial risks also running high if the efficacy of the vaccine was low.
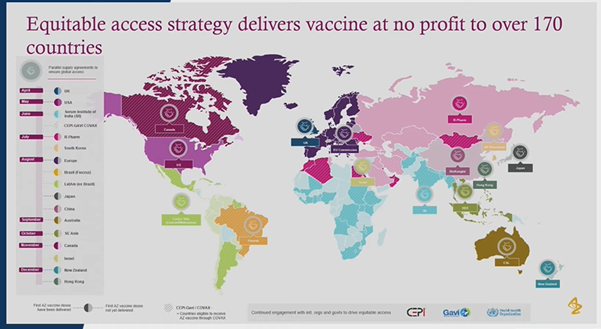
Certain stages of the vaccine development including clinical trials in US, India and Japan were sponsored by AstraZeneca which aided the global effort.
The public lecture was hosted by the Elizabeth Blackwell Institute for Health Research at the University of Bristol as part of their annual lecture series that honours Dr Elizabeth Blackwell.
Bristol Uni announces that all staff and students are now required to wear a mask on campus
Pioneering research shows long COVID in children poorly understood by doctors
Born in Bristol in 1821, Dr Blackwell was the first woman to be listed on the British Medical Register in 1859 making her the first female practising doctor in the UK. Prior to that, she was the first woman to graduate from a medical school in the USA where she moved to as a child. The talk by Prof Dame Gilbert marked 200 years since Dr Blackwell’s birth.
Featured Image: Epigram/Sarah Dalton
If you missed the online lecture from Prof Dame Gilbert you can watch the recording here.
Learn more about Elizabeth Blackwell by following this link to read a short biography.